Most people assume that if two pills have the same active ingredient, they’re the same. That’s not true - and for people with food allergies or sensitivities, this gap can be dangerous. Generic medications are required by the FDA to match the brand-name version in strength, dosage, and active ingredient. But when it comes to what’s inside the pill besides the medicine? That’s a different story.
What Are Inactive Ingredients, and Why Do They Matter?
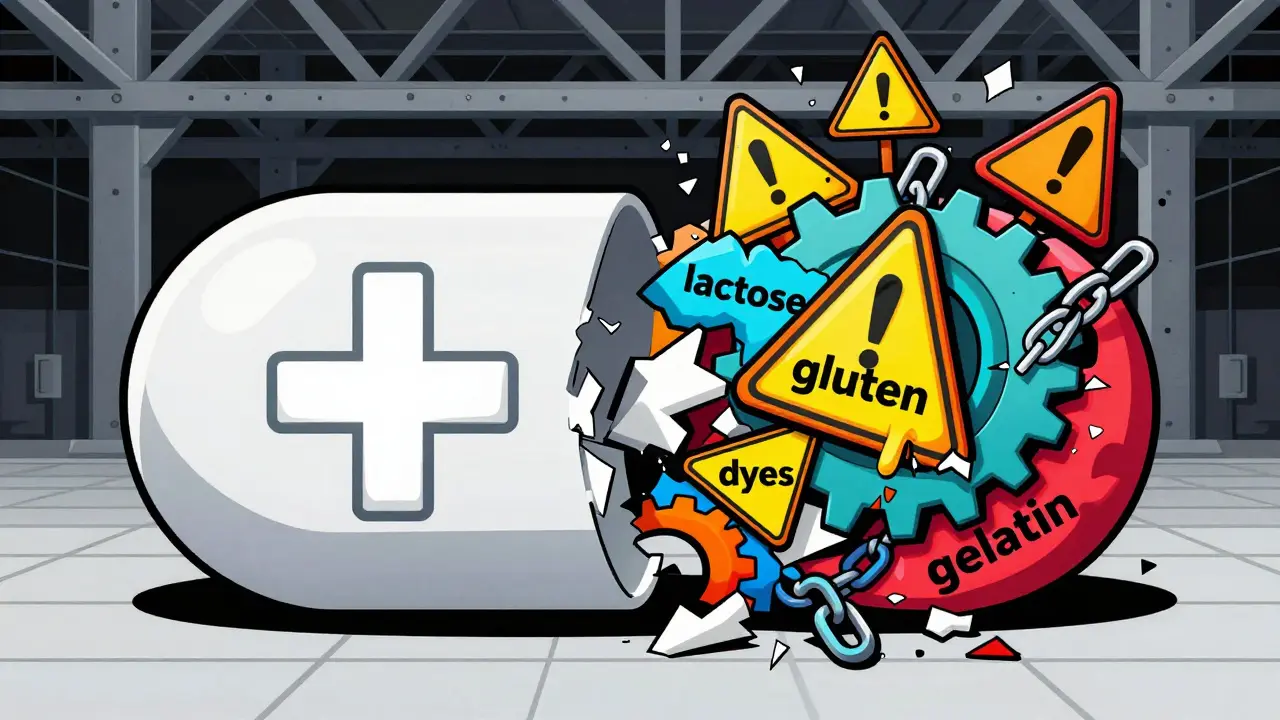
Inactive ingredients - also called excipients - are the non-medicine parts of a pill. They help the drug hold its shape, dissolve properly, taste better, or last longer on the shelf. Think of them as the scaffolding of the pill. But here’s the catch: up to 99% of a pill can be made of these ingredients. In fact, 90% of all oral medications in the U.S. contain at least one ingredient that could trigger a reaction in someone with a sensitivity. Common culprits include:- Lactose (found in over 20% of prescription drugs)
- Gluten (hidden in fillers and coatings)
- Food dyes like FD&C Yellow #5 (tartrazine) and Red #40
- Gelatin (often from pork or beef)
- Soy oil, egg proteins, and milk proteins
- Bisulfites (used as preservatives, can trigger asthma)
- Shellfish-derived ingredients (like chitosan)
Why Generics Can Be Riskier Than Brand-Name Drugs
Brand-name drugs and their generic versions must have the same active ingredient. But the FDA doesn’t require them to have the same inactive ingredients. That means a generic version of your blood pressure pill might contain lactose, while the brand-name version doesn’t. Or vice versa. Take Singulair®: the 10mg tablet has lactose, but the 4mg and 5mg versions don’t. If you switch dosages - even within the same brand - you might accidentally be exposed. And when you switch from brand to generic, you’re often switching to a completely different manufacturer with different fillers. A 2021 Safe Medication report found that 87% of pharmacists have had patients report reactions after switching to a generic. These aren’t just side effects - they’re allergic responses. One patient with Celiac disease developed severe gut inflammation after switching to a generic version of a thyroid med that contained gluten. The active ingredient was identical. The problem? The filler.Why Labeling Is So Confusing (and Often Missing)
You’d think the label would tell you what’s inside. But it doesn’t. The FDA only requires manufacturers to list certain high-risk ingredients - like peanut oil - on the label. Everything else? Optional. Lactose? No mandatory label. Gluten? Not required. Food dyes? Sometimes listed, sometimes not. Bisulfites? Only if they’re above a certain threshold. This creates a dangerous blind spot. In the European Union, all excipients must be listed. Since 2019, that rule has led to a 37% drop in allergic reactions to medications. In the U.S.? No such rule. Even though 68% of major drug companies have improved their labeling since the 2019 MIT study, there’s no law forcing them to do it. And here’s the kicker: the ingredient list on the pill bottle? Often incomplete. The full list is buried in the package insert - the small booklet that comes with your prescription. Most people never read it. And even if they do, the language is technical. “Croscarmellose sodium”? “Magnesium stearate”? What do those even mean?What You Can Do: A Practical Step-by-Step Plan
If you have food allergies, Celiac disease, or suspect you react to medications, here’s what to do:- Know your triggers. Work with an allergist to confirm what you’re sensitive to. Don’t assume - get tested. Lactose intolerance is different from a milk protein allergy. Gluten sensitivity isn’t the same as Celiac disease. Each has different thresholds and risks.
- Build your own list. Write down every ingredient you must avoid. Include hidden forms: lactose = milk sugar, gelatin = animal collagen, starch = might be corn or wheat. Keep this list updated.
- Ask your pharmacist. Don’t rely on the label. Pharmacists have access to full ingredient databases. Ask: “Does this generic version contain [lactose, gluten, soy, etc.]?” Most pharmacists (94%) routinely help patients with this.
- Check the package insert. Look for the “Inactive Ingredients” section. If you don’t get one with your prescription, call the pharmacy and ask for it. Many now offer digital copies via apps or email.
- Use the Inactive Ingredient Finder app. Launched in early 2023 by MIT researchers, this free tool lets you search over 98% of U.S. medications by name and see their full excipient profile. It’s available on iOS and Android.
- Request alternatives. If your current generic has a problem ingredient, ask your doctor for a different generic brand, a brand-name version, or a compounded medication. Compounded drugs are made by specialty pharmacies and can be customized to be allergen-free.
- Track your reactions. If you feel unwell after starting or switching a medication - even if it’s mild - write it down. Note the drug name, dosage, and when symptoms started. Bring this to your doctor. It’s the best way to prove a pattern.
Who’s Most at Risk?
It’s not just people with obvious food allergies. People with:- Celiac disease (gluten-free is critical)
- Severe milk protein allergy (lactose can be a trigger)
- Chronic asthma (bisulfites can cause attacks)
- Irritable Bowel Syndrome (FODMAP sugars in meds can flare symptoms)
- Children (many pediatric meds use dyes and sweeteners)
- Seniors taking five or more pills daily (risk of cumulative exposure)

What’s Changing - And What’s Not
The FDA held a public workshop in 2021 on this issue. In March 2022, they released draft guidance recommending that eight high-risk excipients - including lactose, gluten, soy, sulfites, and azo dyes - be clearly labeled. But as of October 2023, it’s still just a draft. No final rule. Meanwhile, some progress is happening on the ground. Forty-two percent of U.S. pharmacies now use electronic systems that flag allergens in prescriptions - up from 17% in 2020. A few generic manufacturers have started offering allergen-free versions, but only 12% of generics currently do. The IQVIA Institute predicts that by 2027, 30% of new generic medications will offer at least one allergen-free option. That’s promising. But until labeling becomes mandatory, patients are still left to play detective.When to Speak Up
If you’ve had a reaction - even a small one - after switching to a generic, don’t ignore it. Say something. Tell your doctor. Tell your pharmacist. Ask for a different formulation. Your reaction might be rare, but it’s real. And you’re not alone. The American Medical Association called in May 2023 for mandatory labeling of all excipients by 2026. That’s coming. But until then, you have to be your own advocate.Final Thought: You’re Not Overreacting
Some doctors still say, “Allergies to inactive ingredients are rare.” True - they are. But “rare” doesn’t mean “not real.” For the 1 in 1,000 people who have a serious reaction, it’s everything. And if you’re one of them, your health isn’t a statistical footnote. It’s your life. Don’t wait for the system to catch up. Know your ingredients. Ask questions. Demand clarity. Your medication should heal you - not hurt you.Can generic medications cause allergic reactions even if they have the same active ingredient as the brand-name version?
Yes. Generic medications must match the brand-name version in active ingredient strength and effectiveness, but they can use different inactive ingredients. These fillers - like lactose, gluten, dyes, or gelatin - can trigger allergic reactions or intolerances in sensitive individuals. A patient might react to a generic version of their medication even though the medicine itself is identical.
How do I find out what inactive ingredients are in my medication?
Check the package insert that comes with your prescription - it’s usually a small booklet. Look for a section titled “Inactive Ingredients.” If you don’t have it, call your pharmacy and ask for a copy. You can also use the free Inactive Ingredient Finder app (launched in 2023 by MIT), which has data on 98% of U.S. medications. For over-the-counter drugs, the ingredients are listed on the Drug Facts label.
Is lactose in medications a problem for people with lactose intolerance?
For most people with lactose intolerance, the small amount of lactose in a pill (usually under 100 mg) won’t cause symptoms. But for those with severe milk protein allergy - not just lactose intolerance - even trace amounts can trigger a reaction. Milk protein (casein, whey) is the allergen, not lactose. If you have a confirmed milk protein allergy, avoid any medication containing lactose, as it’s often derived from milk and may contain residual proteins.
Are there gluten-free generic medications available?
Yes, but they’re not labeled as gluten-free unless the manufacturer chooses to certify them. The Celiac Disease Foundation says only about 15% of commonly prescribed drugs have been verified as gluten-free. You need to check the inactive ingredient list manually. Some pharmacies and compounding labs can source or make gluten-free versions upon request.
Can I ask my pharmacist to switch me to a different generic version without changing my doctor’s prescription?
Yes. Pharmacists can often substitute one generic manufacturer for another without needing a new prescription - as long as the active ingredient and dosage are the same. If you’re reacting to one generic, ask your pharmacist to try a different brand. Many pharmacies keep multiple generic suppliers on hand and can switch you quickly.
What should I do if I suspect I’m having a reaction to an inactive ingredient?
Stop taking the medication and contact your doctor immediately. Document the symptoms, when they started, and which medication you took. Bring the pill bottle and package insert to your appointment. Your doctor may refer you to an allergist for testing. In the meantime, avoid that medication and any similar generics until you know what caused the reaction.
Are compounded medications a safer option for people with allergies?
Compounded medications, made by specialty pharmacies, can be customized to exclude allergens like lactose, gluten, dyes, or gelatin. They’re not FDA-approved like mass-produced drugs, but they’re regulated by state pharmacy boards and are often used for patients with complex allergies. Ask your doctor if a compounded version is appropriate for your needs.
Why don’t drug companies use the same inactive ingredients in generics and brand-name drugs?
Generic manufacturers aim to produce the drug at the lowest possible cost. They often use cheaper, widely available fillers - like lactose or corn starch - that aren’t always the same as the brand-name version. There’s no legal requirement for them to match excipients, so they choose what’s most economical. This saves money but creates risks for sensitive patients.




Okay but like... why is this even a thing? I switched to a generic thyroid med last year and got a rash that lasted three weeks. My doctor just shrugged and said 'it's probably stress.' Turns out it was gluten in the filler. No one told me. No one even asked. We need labels. Like, now.
January 2Also, the Inactive Ingredient Finder app? Life saver. I use it before every refill now. Seriously, download it.
Sally Denham-Vaughan