Why stability testing isn’t just paperwork-it’s patient safety
Every pill, injection, or inhaler you take has a hidden clock. It starts ticking the moment it leaves the manufacturing line. Stability testing is how manufacturers track that clock-making sure the medicine you buy today will still work safely a year, two years, or even five years from now. This isn’t optional. It’s the legal and scientific backbone of every FDA-approved drug. Without it, you could be taking a tablet that’s lost half its potency, or worse, one that’s turned toxic.
In 2021, nearly 1 in 6 drug recalls in the U.S. traced back to stability failures: degraded active ingredients, unexpected chemical byproducts, or packaging that let moisture in. These aren’t theoretical risks. They’re real, documented events that led to hospitalizations and delayed treatments. Stability testing exists to catch those problems before they reach you.
How stability testing actually works
It’s not guesswork. It’s controlled science. Drug manufacturers place sealed samples of their product-capsules, vials, syringes-into environmental chambers that mimic real-world conditions. For most drugs, that means 25°C (77°F) and 60% humidity, the standard for temperate climates. For regions with hotter, wetter weather, like Southeast Asia or parts of Latin America, they test at 30°C and 65% humidity.
Every few months-typically at 0, 3, 6, 12, 24, and 36 months-they pull out samples and run tests. They check:
- Appearance: Has the color changed? Is it crumbling or sticking together?
- Chemical strength: Is the active ingredient still at 90-110% of the labeled amount?
- Degradation products: Are any new chemicals forming? Are they below safety limits?
- Dissolution: Does the tablet still break down properly in the body?
- Microbial growth: Is the product still sterile? No mold, no bacteria.
All these tests use validated methods-usually HPLC or mass spectrometry-that can detect even tiny changes. And they don’t just test one batch. They test multiple batches, across different production runs, to make sure the results aren’t a fluke.
Accelerated testing: the shortcut that isn’t really a shortcut
Waiting three years to find out if a drug stays stable isn’t practical for bringing new medicines to market. That’s why companies run accelerated tests: 40°C and 75% humidity for six months. Think of it like a stress test. If a drug breaks down fast under these harsh conditions, it’s a red flag.
But here’s the catch: accelerated testing can’t predict everything. A 2021 study in the Journal of Pharmaceutical Sciences showed that while it catches 80% of potential issues, the remaining 20% only show up under real-time conditions. That’s why regulators still require long-term data. Accelerated results help you plan-but real-time data gives you the final answer.
For complex drugs like biologics-those made from living cells-accelerated testing is even less reliable. These drugs can degrade in ways that don’t follow predictable patterns. That’s why companies developing them often run real-time studies from day one.
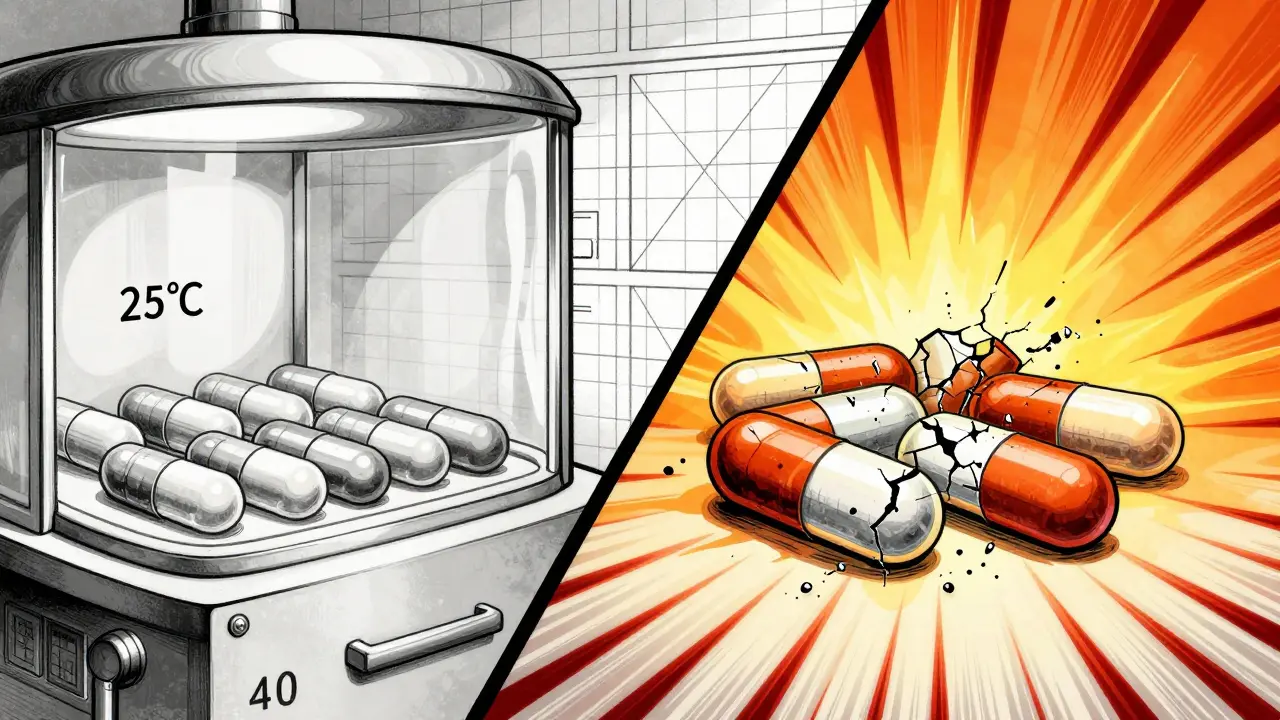
What happens when stability testing fails
Failure isn’t just about a delayed launch. It’s about trust.
In 2021, the FDA issued a warning letter to a manufacturer of a cancer drug after they ignored out-of-specification (OOS) results from their stability tests. Instead of investigating why potency dropped, they just retested until they got a passing number. That’s called data manipulation-and it’s a direct violation of cGMP rules. The approval was delayed by 14 months. The company lost millions. And patients waited longer for a treatment they needed.
On the flip side, stability testing has prevented disasters. Between 2020 and 2022, the International Pharmaceutical Aerosol Consortium reported that 47 drug products were blocked from entering the market because stability tests revealed dangerous degradation products. One was an inhaler that produced a toxic compound when exposed to heat. Another was a liquid antibiotic that turned cloudy and lost effectiveness after six months. Both were caught before a single bottle reached a pharmacy.
Costs, challenges, and how companies cope
Stability testing isn’t cheap. A single product study can cost between $50,000 and $150,000. A large company with dozens of products might spend over $2 million a year just on stability chambers, testing, and staff. That’s why 72% of pharmaceutical companies outsource at least part of this work to contract labs like SGS, Eurofins, or Charles River.
But even outsourcing comes with headaches. Temperature excursions in stability chambers are the #1 problem. One Reddit user, a stability technician, shared that humidity spikes in their lab caused a three-month data gap-delaying their drug approval by eight months and costing $2.3 million in lost sales. Chambers need quarterly temperature mapping, calibrated by ISO 17025-accredited labs. Each mapping costs about $8,500.
Another big challenge is data management. ICH guidelines require companies to keep stability records for at least one year after the product expires. For a drug with a five-year shelf life, that’s six years of raw data. Paper records are a nightmare. Companies using electronic systems cut review time by 55%. But setting up those systems takes 6-9 months of validation work.

The future: smarter, faster, and more risk-based
Stability testing is changing. The big shift? Moving from blanket testing to smart testing.
ICH Q12, introduced in 2018, lets companies use science and data to reduce unnecessary testing. A mid-sized generics company reported cutting their stability samples by 40% and saving $120,000 per product annually by using this approach. They didn’t skip testing-they focused it on what mattered most.
Then there’s continuous manufacturing. Traditional batch testing assumes each batch is identical. But with continuous manufacturing-where drugs are made in a steady stream-each moment is unique. The new ICH Q13 guideline, finalized in February 2023, requires real-time stability monitoring during production. This is a game-changer.
And soon, AI will play a bigger role. By 2027, experts predict machine learning models will predict degradation paths with 30-40% less testing. Instead of waiting 24 months to see if a drug degrades, companies might simulate it in weeks using data from thousands of past studies.
But here’s the truth: no matter how smart the tools get, stability testing won’t disappear. Why? Because patients deserve to know their medicine won’t fail them. And regulators won’t let that risk go unchecked.
What you should know as a patient
You don’t need to understand HPLC or ICH guidelines. But you should know this: expiration dates aren’t arbitrary. They’re based on real data from real testing. If your medicine is past its date, don’t take it-even if it looks fine. Potency loss isn’t always visible.
And if you’re using a generic drug, remember: it went through the same stability testing as the brand-name version. The FDA requires it. That’s why generics are safe and effective.
Stability testing is invisible. You never see it. But every time you take a pill and it works exactly as it should, that’s the result of thousands of hours of lab work, meticulous records, and disciplined science. It’s not glamorous. But it’s essential.
What is the purpose of stability testing in pharmaceuticals?
Stability testing determines how a drug’s quality changes over time under real-world conditions like heat, humidity, and light. It ensures the medicine remains safe, effective, and within approved specifications until its expiration date. This data is used to set shelf life, storage instructions, and packaging requirements.
How long does stability testing take?
Real-time stability testing typically runs for 24 to 36 months, with samples tested at regular intervals (0, 3, 6, 12, 18, 24, 36 months). Accelerated testing (40°C/75% RH) runs for 6 months and helps predict long-term behavior, but it doesn’t replace real-time data for final shelf-life determination.
Are stability tests required by law?
Yes. Regulatory agencies like the FDA and EMA require comprehensive stability data as part of all New Drug Applications (NDAs) and Abbreviated New Drug Applications (ANDAs). Without this data, a drug cannot be approved for sale in the U.S., EU, or other major markets.
What happens if a drug fails a stability test?
If a drug fails, the manufacturer must investigate the cause. If the issue is confirmed, the product may be recalled, its shelf life shortened, or its approval delayed. In severe cases, like data manipulation or failure to investigate, the FDA can issue warning letters, block approvals, or even shut down production.
Do all types of drugs need stability testing?
Yes. All pharmaceutical products-small molecule pills, injectables, biologics, inhalers, and even topical creams-require stability testing. Biologics and complex formulations are especially sensitive and often require more frequent and detailed testing due to their susceptibility to degradation.
Can stability testing be shortened or simplified?
Yes, under ICH Q12 guidelines, companies can use risk-based approaches and prior knowledge to reduce testing for well-characterized products. For example, if a drug’s chemistry and packaging are proven stable over time, fewer samples or test points may be needed. But this requires strong scientific justification and regulatory approval.
What’s the difference between stability testing and quality control?
Quality control checks each batch at the time of release to ensure it meets specifications. Stability testing tracks how the product changes over time under storage conditions. QC says, “Is this batch good today?” Stability says, “Will it still be good six months from now?” Both are essential.
How do I know if my medicine’s stability data is reliable?
You can’t see the data, but you can trust the system. The FDA inspects stability labs, requires validation of testing methods, and reviews all stability data before approving a drug. If a company falsifies data, they face severe penalties. The system isn’t perfect, but it’s rigorously monitored.




Stability testing is the unsung hero of pharmacology - like the accountant who makes sure your tax return doesn’t get you jailed. HPLC, mass spec, environmental chambers… it’s not sexy, but without it, your ‘miracle drug’ could be a slow poison. I’ve seen labs where humidity spikes turned a $2M batch into science fiction. The real win? When the data says ‘no’ - that’s when lives are saved.
December 30Aayush Khandelwal