When you're diagnosed with IgA Nephropathy, the first question that comes to mind isn't about symptoms or lab results-it's how bad will it get? And more importantly, can it be stopped? The answer isn't simple, but the latest guidelines from KDIGO in 2025 have reshaped everything we thought we knew about this condition. IgA Nephropathy, also called Berger's disease, is the most common cause of primary glomerulonephritis worldwide. It happens when your immune system mistakenly sends IgA antibodies to your kidneys, where they build up, trigger inflammation, and slowly damage the filtering units. Left unchecked, it can lead to kidney failure. But now, for the first time, we have real, evidence-based tools to change that outcome.
What’s the Real Risk of Progression?
It’s easy to panic when you hear "kidney disease," but not everyone with IgA Nephropathy ends up on dialysis. Studies show that about 30% to 40% of patients will develop end-stage kidney disease (ESKD) within 10 to 20 years. That sounds scary, but here’s the key: it’s not inevitable. The biggest predictor of how fast the disease progresses isn’t just how much blood is in your urine-it’s how much protein you lose through your kidneys. Proteinuria is the red flag doctors watch most closely.
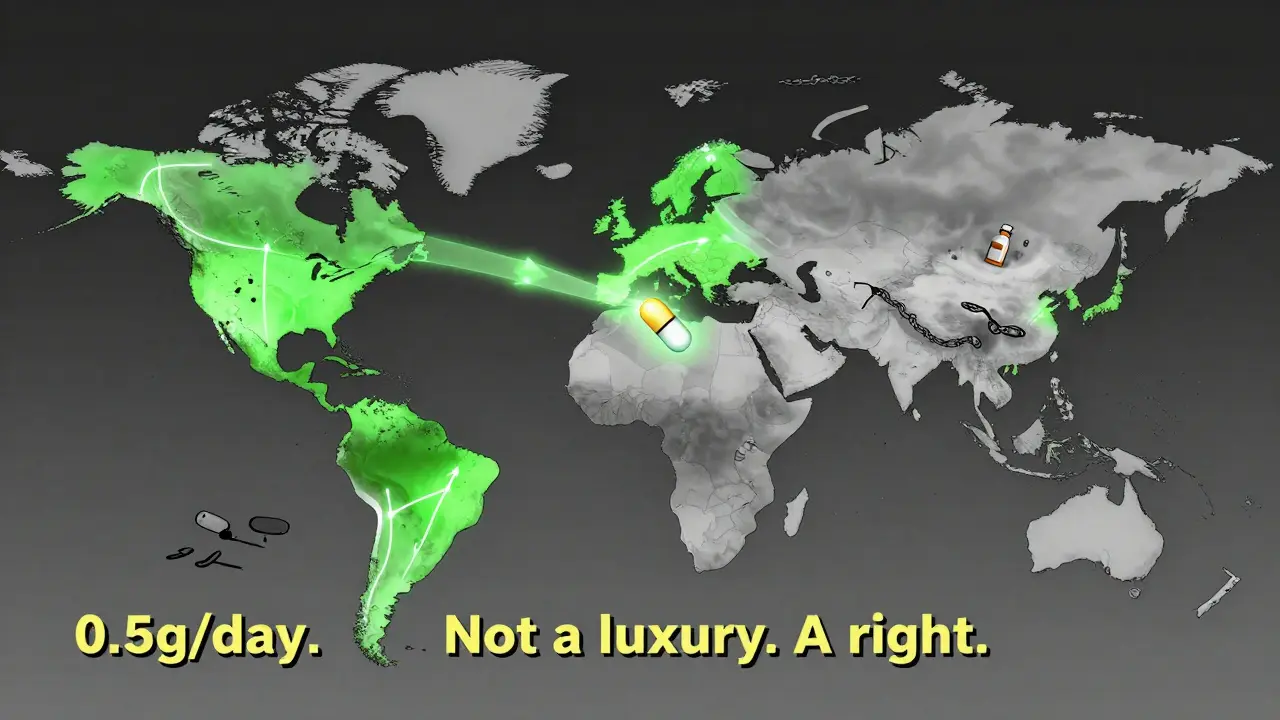
Older guidelines said keeping protein under 1 gram per day was good enough. But new data from Cleveland Clinic in 2025 showed that even patients with proteinuria as low as 0.44 to 0.88 grams per gram of creatinine still had a 30% chance of kidney failure within 10 years. That’s why KDIGO 2025 moved the goalpost: the new target is under 0.5 grams per day. It’s a tougher standard, but the science says it matters. Every 0.1 gram reduction in protein loss lowers your risk of kidney failure by about 15%. This isn’t just theory-it’s backed by real-world outcomes from global registries.
The New Treatment Paradigm: Stop Waiting, Start Acting
For years, the standard approach was "wait and see." You’d start with blood pressure meds-usually ACE inhibitors or ARBs-and wait three months to see if proteinuria improved. If it didn’t, you’d move to steroids or other immunosuppressants. But here’s the problem: while you were waiting, your kidneys kept getting damaged. That’s like putting a bandage on a leaky pipe while the water keeps flowing.
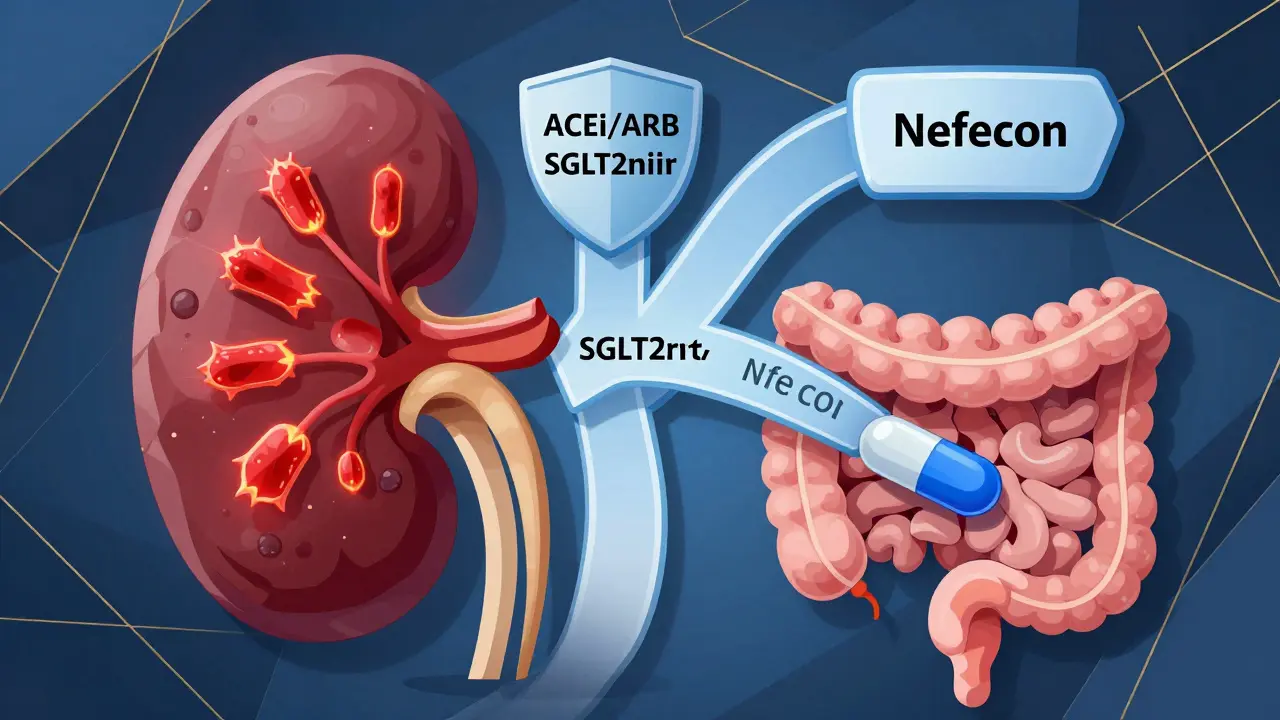
KDIGO 2025 flipped the script. Now, if you’re at high risk-meaning you have persistent proteinuria over 0.75 g/day, high blood pressure, or abnormal kidney biopsy findings-you don’t wait. You start two treatments at the same time. One targets the immune system’s attack on your kidneys. The other protects your kidneys from the damage caused by high pressure and protein leakage.
This dual approach isn’t guesswork. It’s based on clinical trials that showed patients who got combination therapy early had significantly slower decline in kidney function. The old "sequential" method is now considered outdated. As Dr. Laura H. Mariani from the University of Michigan put it, "Sequential therapy allowed continued disease activity during the initial supportive care period. We can’t afford that anymore."
What Treatments Are Actually Used Today?
There are now four main pillars of IgA Nephropathy treatment-and they’re often used together:
- RAS inhibitors (ACEi or ARBs): These are the foundation. They lower blood pressure and reduce protein leakage. Most patients start here, but now they’re paired with other drugs right away.
- SGLT2 inhibitors (like dapagliflozin or empagliflozin): Originally for diabetes, these drugs have shown surprising benefits in kidney disease. They reduce proteinuria, lower blood pressure, and slow kidney decline-even in non-diabetics. They’re now recommended for nearly all high-risk patients.
- Nefecon: This is the first drug specifically approved for IgA Nephropathy. Approved by the FDA in December 2023, it’s a targeted-release budesonide that acts in the gut to stop the production of abnormal IgA antibodies. It’s not a steroid you swallow-it’s designed to work where the problem starts. Patients report fewer side effects than traditional steroids.
- Systemic glucocorticoids (like prednisone): Still used, but now only for patients who can’t access or tolerate Nefecon. They’re powerful but come with risks: weight gain, diabetes, bone loss, mood changes. The goal is to use the lowest dose for the shortest time possible.
There are also region-specific treatments. In Japan, tonsillectomy is common because infections there often trigger IgA flares. In China, mycophenolate mofetil and hydroxychloroquine show strong results. But outside those regions, the evidence isn’t strong enough to recommend them routinely.

Why Some Patients Struggle to Get Treatment
Even with better guidelines, access isn’t equal. Nefecon costs $125,000 a year in the U.S. Many insurance companies deny coverage at first-68% of patients in a 2025 survey reported initial denials. It takes appeals, paperwork, and sometimes months to get approved. Meanwhile, patients are losing kidney function.
Global disparities are even starker. In high-income countries, 85% of patients get guideline-recommended care. In low- and middle-income countries, that number drops to 22%. The same drugs that save kidneys in Manchester or Boston aren’t available in rural India or sub-Saharan Africa. And while new drugs like sparsentan (a dual endothelin receptor antagonist) are now approved in Europe and the U.S., they’re still out of reach for most of the world.
Patient surveys from the IgA Nephropathy Foundation show that 83% of people care more about preserving quality of life than just slowing kidney decline. That’s why treatment burden matters. One Reddit user, "IgAN_Mom," described managing four medications with complex schedules as "overwhelming for my 16-year-old." Simultaneous therapy is more effective-but it’s also more complicated. That’s why doctors now use decision aids and electronic health record tools to help patients understand their options.
Monitoring and What Comes Next
Once treatment starts, you’ll be monitored closely. Monthly checks for proteinuria and blood pressure are standard for the first three months. After that, quarterly visits are typical. A kidney biopsy isn’t done every year-it’s usually only needed at diagnosis or if things suddenly worsen. The Oxford Classification (MEST-C score) from your biopsy helps predict risk, but it’s not used to change treatment on its own. It’s one piece of the puzzle.
The future is coming fast. Fifteen phase 3 trials are currently active. One called TARGET-IgAN (NCT05921545) is testing whether biomarkers can predict who will respond to which drug. Imagine a blood test that tells your doctor: "You’ll respond to Nefecon but not steroids." That’s the goal. Dr. Jonathan Barratt predicts that within five years, treatment will be chosen by biomarker profile, not just protein levels or biopsy results.
But even with all this progress, the big question remains: How low should we push proteinuria? We don’t yet know if aiming for under 0.3 g/day is safe or beneficial. No trial has tested that yet. For now, 0.5 g/day is the target. And while we wait for more data, the message is clear: early, combined, aggressive treatment saves kidneys.
What Patients Are Saying
Online communities are full of real stories. "GFR_Warrior" on Reddit wrote in March 2025: "The 90-day wait for immunosuppression before felt like watching my kidney function decline unnecessarily." That sentiment helped push KDIGO toward simultaneous therapy. Others praise Nefecon: 72% of 150 surveyed members said it had fewer side effects than traditional steroids.
But the emotional toll is real. The uncertainty of whether your kidneys will hold out, the fear of dialysis, the frustration of insurance battles-it all adds up. That’s why patient-centered care is now part of the guidelines. Your age, your other health conditions, your tolerance for side effects, your access to care-all of it matters. There’s no one-size-fits-all. Your doctor should be talking to you, not just at you.
Can IgA Nephropathy be cured?
There’s no cure for IgA Nephropathy yet. But with the right treatment, many patients can stop or very significantly slow the progression of kidney damage. The goal isn’t to eliminate the disease-it’s to prevent kidney failure. With early, combined therapy, a large number of patients can live full lives without ever needing dialysis or a transplant.
How long does it take for treatment to work?
It takes time. Proteinuria doesn’t drop overnight. Most patients see a reduction in protein loss after 3 to 6 months of consistent treatment. Nefecon may show effects as early as 3 months, while SGLT2 inhibitors and RAS blockers often take 4 to 6 months. The key is consistency. Missing doses or stopping early can undo progress. Doctors recommend continuing treatment even if lab results improve.
Are steroids still used for IgA Nephropathy?
Yes-but not as the first choice anymore. Systemic steroids like prednisone are still used, especially if Nefecon isn’t available or if a patient can’t tolerate it. But because of side effects-weight gain, high blood sugar, bone loss, mood swings-doctors now prefer Nefecon or SGLT2 inhibitors when possible. Steroids are typically given at the lowest effective dose for the shortest time, often tapered over 6 to 9 months.
What happens if I don’t treat IgA Nephropathy?
Without treatment, about 30% to 40% of people will develop end-stage kidney disease within 10 to 20 years. That means needing dialysis or a kidney transplant. But it’s not guaranteed. Some people have slow progression, especially if they control their blood pressure and avoid smoking or NSAIDs. Still, untreated IgA Nephropathy carries a high risk of irreversible kidney damage. Early treatment reduces that risk by up to 60%.
Is diet important for managing IgA Nephropathy?
Yes, but not in the way most people think. There’s no special "IgA diet," but limiting salt is critical-too much salt makes blood pressure harder to control and increases protein leakage. A low-sodium diet (under 2,300 mg per day) is recommended. Protein intake doesn’t need to be severely restricted unless kidney function is already very low. Avoiding processed foods, sugary drinks, and excessive alcohol helps overall kidney and heart health. Always work with a renal dietitian to tailor your plan.
Can I still exercise with IgA Nephropathy?
Absolutely. Regular, moderate exercise helps control blood pressure, reduces inflammation, and improves overall well-being. Walking, swimming, cycling, and light strength training are all encouraged. Avoid heavy weightlifting or extreme endurance sports if your proteinuria is high or your eGFR is low. Always check with your nephrologist before starting a new routine, especially if you’re on immunosuppressants.
What’s Next for IgA Nephropathy?
The next five years will be transformative. New drugs targeting the complement system and APRIL (a protein involved in IgA production) are in late-stage trials. Vera Therapeutics and other biotechs are racing to bring these to market. The focus is shifting from broad immunosuppression to precision medicine-finding the right drug for the right patient based on their biology, not just their symptoms.
But technology alone won’t fix this. Access, cost, and equity are the real challenges. A treatment that works in a clinic in London won’t help someone in rural Nigeria if it’s unaffordable or unavailable. The KDIGO guideline ends with a clear call: "The goal remains simple but ambitious: delay and prevent kidney failure across an entire lifetime, while minimizing treatment burden and toxicity."
That goal is within reach-but only if we make sure it reaches everyone, not just those with the best insurance or the closest specialist.